Applications of RepliGut® Models
RepliGut® Models mimic in vivo responses to support a variety of research and pharmacological applications.
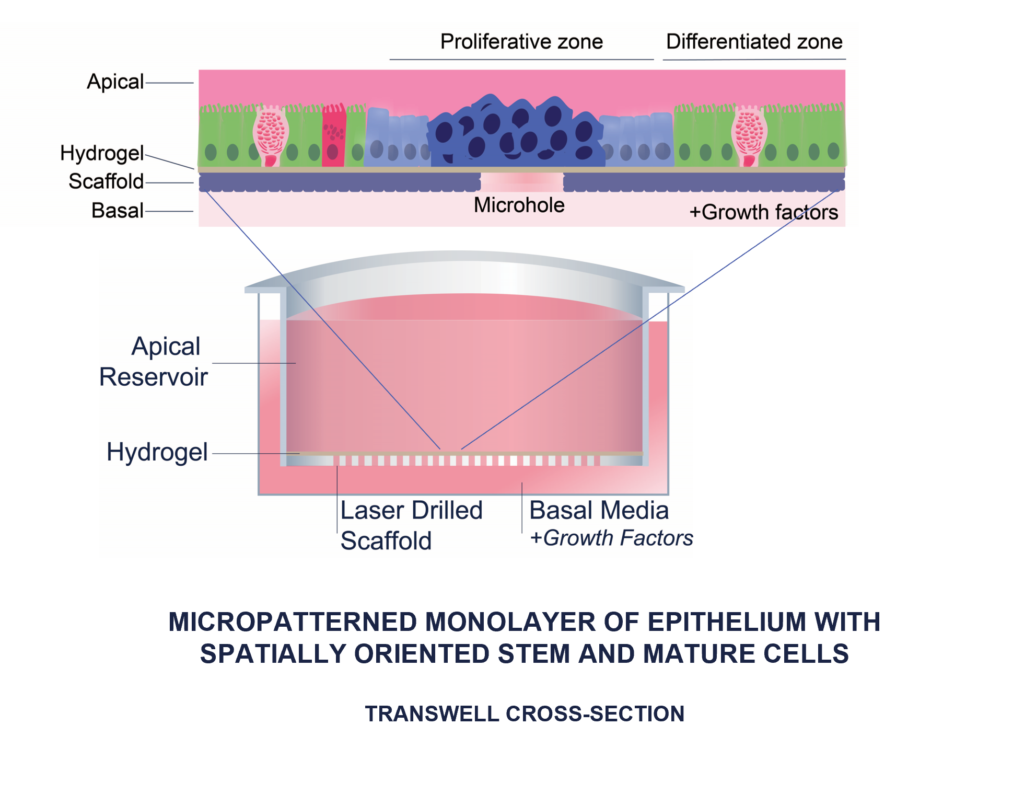
Why RepliGut® Planar?
RepliGut® Planar models recreate the biology of the human intestine using a convenient membrane supported culture platform. These models represent the different regions of the intestine enabling physiologically relevant and translatable study of inflammation, toxicity, bioavailability, barrier integrity, and host-microbe interactions.

Clinically relevant studies powered by RepliGut® Models
There is a lack of easy-to-use in vitro models that can accurately simulate in vivo gastrointestinal (GI) responses. Results from cell-based assays (Caco-2) and animal models often fail to translate into clinical applications.
RepliGut® Models recreate the biology of the human intestine and enable physiologically relevant and translatable study of inflammation, GI toxicity, pharmacokinetics (ADME), and host-microbre interactions.

Applications of RepliGut® systems
The standardized plate format with access to both apical and basal surfaces enables wide range of assays to suit your needs.

Inflammatory Bowel Disease
Chronic inflammation of the GI tract is implicated in a number of human diseases, such as Inflammatory Bowel Disease (IBD). Over time, inflammation can damage the epithelial tight junctions that maintain the GI barrier integrity, leading to increased gut permeability, systemic inflammation, and other adverse reactions.
RepliGut® Models can be stimulated to induce an inflammation response using pro-inflammatory cytokines, like tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-y), to assess intestinal barrier responses and the efficacy of anti-inflammatory drugs.

Using a RepliGut® Model to predict anti-inflammation drug efficacy
- A RepliGut® Model was used to asses intestinal response to Tofacitinib, an anti-inflammatory drug used to treat ulcerative colitis
- Barrier function was stimulated with IFN-y treatment
- 3 different donor models were used to incorporate generic variability for a more rigorous evaluation
GI Toxicity
GI toxicities are one of the most common adverse events during Phase 1 clinical trials. In vitro cytotoxicity assays are used to predict drug-induced GI toxicity often include Caco-2 cells that fail to represent the biology of the human gut.
RepliGut® Planar is a human stem-cell derived model which, unlike spherical organoids, offers independent access to apical and basal surfaces and the ability to directly measure barrier disruption.


ADME
(Absorption, Distribution, Metabolism, Excretion)
In vitro ADME studies are used to screen drug candidates for key physical and chemical properties that drive their absorption, distribution, metabolism, and excretion by the body. Absorption assays are often performed using Caco-2 cells which lack many active transporters and metabolic enzymes that contribute to clinical bioavailability.
RepliGut® Models are derived from human adult donor stem cells obtained from multiple regions of the gut. This enables researchers to assess distinct regions of the small intestine and colon, from more than one donor, for a more thorough vetting of drug candidate bioavailability.
Host-Microbe Interactions
Evidence suggests that human health is highly dependent upon a balanced relationship with microorganisms, and when this host-microbe balance is compromised, infection and disease can result. A healthy intestinal epithelium provides a protective barrier between the microbe and the host.
RepliGut® Model microbiome studies, including the testing of microbial supernates from different populations of microbes to assess localized impacts on the intestinal epithelium.
Gut Hormone and Metabolism Research
RepliGut® recreates the architecture and function of the human intestinal epithelium, making it a valuable tool for investigating gut hormone biology, including GLP-1, in vitro. By modeling the cellular diversity and barrier properties of the intestine, RepliGut® provides a more human-relevant platform than animal systems for exploring hormone secretion, transport, and metabolic pathways.
Researchers are applying RepliGut® to studies in areas such as diabetes, obesity, and the gut–brain axis, where gut-derived hormones like GLP-1 play a central role.
Key Advantages:
- Human intestinal monolayers that support evaluation of hormone activity
- In vitro system to examine secretion, stability, and transport mechanisms
- Scalable format suitable for drug discovery and mechanistic studies
Key Applications

Chronic inflammation of the GI tract is implicated in a number of human diseases such as inflammatory bowel diseases (IBD). Over time, inflammation can lead to increased gut permeability, systemic inflammation, and other adverse reactions.
RepliGut® Models can be stimulated to induce an inflammation response that impacts intestinal barrier integrity enabling study of the efficacy of anti-inflammatory drugs.

GI toxicities are the most common adverse event associated with therapeutics. Historically, GI toxicity is poorly anticipated in preclinical development due to lack of predictive model systems.
RepliGut® Models are cultured in a convenient platform that enables testing for toxicity to both proliferative and post-mitotic cell lineages using treatment paradigms on either apical or basolateral surfaces.

Intestinal absorption assays are often performed using the Caco-2 cell model, which lack many of the active transporters and metabolic enzymes that contribute to clinical bioavailability.
RepliGut® Models are cultured in a convenient platform that enables testing for toxicity to both proliferative and post-mitotic cell lineages using treatment paradigms on either apical or basolateral surfaces.
Key Features of RepliGut® Planar
The standardized plate format with access to both apical and basal surfaces enables wide range of assays to suit your needs.
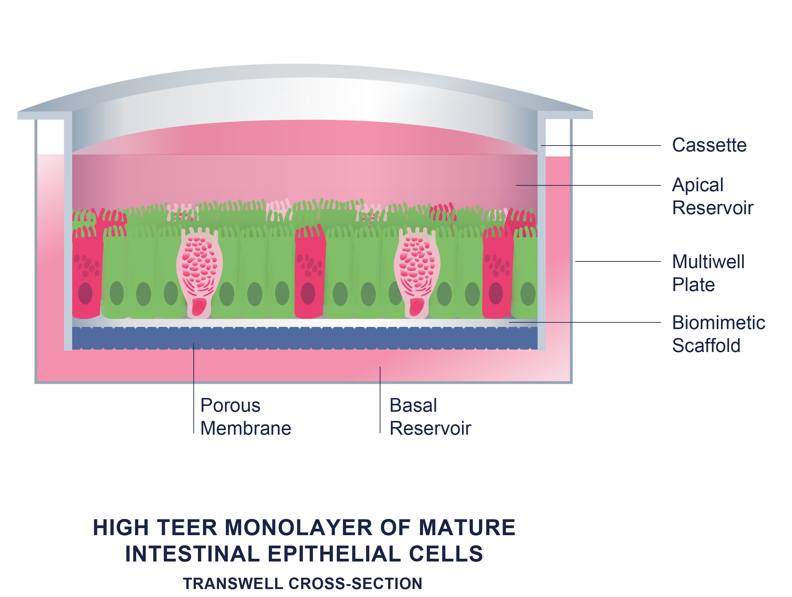
RepliGut® Kits
The RepliGut® kits come in two convenient sizes, 12 or 96 wells, tailored to meet the specific needs of scientists. These comprehensive kits provide everything scientists require to cultivate cells within their own facility, featuring a precoated membrane support multiwell plate, region-specific stem cells, and culture media.
Customer Focused
Quick replies


Multiple Donor Biobank
Unrivaled biobank with multiple donors, allows customers to choose regional specificity of the small intestine and colon from donors of different demographic backgrounds to further enhance study data. Fit-for-purpose specimens, aligned with scientific objectives, and the guaranteed ability to work with the same donor for years.
Smiles guaranteed
World class service
Two ways to use RepliGut® Systems
Enlist Altis scientists to run your projects, or buy kits to set up RepliGut® Planar model in your lab
Kits
RepliGut® Planar Kits are available in both 12-well and 96-well transwell plate formats to accomodate scientist's needs. Kits are sold complete with the cells, plates and media needed to culture this unique human intestinal epithelial model in your own facility. Technical support is available.
RepliGut® Crypt is not yet available in kit format
Services
All service projects for RepliGut® Planar and Crypt are led by scientists with extensive experience in advanced cell culture systems. Assays are customizable based on your needs and can be run using standard or customized cell culture conditions.
Key Features of RepliGut® Planar
The standardized plate format with access to both apical and basal surfaces enables wide range of assays to suit your needs.
Different demographic backgrounds to further enhance study data.
Ability to work with the same donor for years.
Multiple regions available from small intestine and colon.
Ethics Committee approved organ procurement.
Ready to Go With Your Gut?
Contact us to learn more about RepliGut® Model Kits and Services.